Phases
Phase 1: Transfer and release
The physical transfer of live animals requires a huge amount of planning. The source population needs to be large (able to sustain harvest) and robust (genetically diverse). When capturing animals for transfer, you need to maximise genetic diversity wherever possible as this could affect the persistence of your population or its utility as a source for future translocations.
When carrying out the transfer, you will need a skilled team alongside you who are familiar with capture techniques for the species, can provide husbandry and disease management, are able to construct transfer boxes, and perhaps can supply post-release support through the provision of nest boxes or supplementary food.
Find best practice guidelines for several commonly translocated species.
Phase 2: Initial or short-term survival
Following release, it is important that as many founders as possible survive so that the new population can increase quickly and maintain its genetic diversity. In New Zealand, predators, habitat quality and dispersal opportunities are likely to have the largest impact on survival. Post-release monitoring will provide you with important information about how many of the animals you released have survived and contributed to the new population.
Phase 3: Evidence of reproduction
You will need to carry out post-release monitoring to measure the recruitment of offspring produced at the release site, which indicates that animals are able to reach breeding condition, find mates and suitable nesting sites, and successfully breed.
Phase 4: Population growth (medium-term success)
Population growth is critical to the survival of your translocated population. Large populations are better able to withstand adverse events such as extreme weather, predator irruptions or disease outbreaks, and also retain more genetic diversity. Population growth is occurring if you detect more animals than you initially released (including founders) during a defined monitoring period. However, you also need to obtain evidence of ongoing population growth that is consistent with the size of the available habitat.
Phase 5: Viable population (long-term success)
A viable population is one which is large (hundreds of individuals) and has a good level of genetic diversity. Your translocated population can be considered viable if you consistently detect a high number of individuals in each monitoring period. At this stage, there is a low probability of extinction due to chance events.
Translocation factors explained
Here, we explain the various technical terms that are used throughout this material.
Founder number for maximising genetic diversity
The ideal founder number is the number of translocated animals that will be able to establish a viable, genetically diverse population.
This varies between species and release sites but, as a general guideline:
- c. 40 founders will retain 90% of the rare alleles in your translocated population
- fewer founders, e.g. c. 20–40 individuals, may only retain 70–90% of the rare alleles – which may be considered acceptable in some cases
- smaller numbers of founders (< 20 individuals) will have a lower probability of establishing and will retain much less genetic diversity in the long term
When thinking about founders, it is important to remember that these individuals must survive and breed to contribute to the new population. Therefore, you may decide to release more individuals than the desired founder number to allow for some losses due to dispersal, predation and other unpredictable events.
You can release the total number of founders in a single translocation (which is preferable for many species) or over several translocations (multiple years), with the optimal approach depending on your target species and release site.
Because survival and dispersal can vary between species and sites, post-release monitoring is essential to determine the outcome of your translocation.
Factor 1: Habitat connectivity
What is connectivity?
Connectivity relates to the distance of your protected habitat from other similar habitats and the ability of your species to cross unsuitable habitat, e.g. water, farmland, suburbia.
For example, we know that juvenile North Island robins are unwilling to cross 110 m of unsuitable habitat such as farmland but adult South Island robins can cross up to 1 km of water.
A site which might be low connectivity for one species, can be highly connected for species due to differences in dispersal abilities. For example, North Island robins will be isolated on Kapiti Island and at Maungatautari, but North Island kākā will easily disperse out of both sites
There are many gaps in our knowledge of how connectivity affects translocation success. However, it appears that translocations are more successful at sites with low or medium connectivity rather than at very highly connected sites.
Levels of connectivity
High (adjoining)
These sites contain adjoining and usually large areas of suitable habitat, e.g. Ark in the Park, which is 2000 ha of protected habitat within c. 17 000 ha of largely unprotected habitat. When selecting a release site, large sites may intuitively seem better than smaller sites – however, this is not always the case. During the establishment phase, newly translocated animals often disperse widely (and often leave the area you are protecting from predators) and later on offspring can also easily disperse and leave the protected area. In addition, large sites with high connectivity are harder to protect as pests can easily reinvade from surrounding unprotected habitats.
Medium
These sites are within dispersal distance of other suitable habitat but the species must cross unsuitable habitat to reach it. Medium connectivity reduces the probability of dispersal because some animals will be more reluctant to cross unsuitable habitat even if they are able to. For example, several species have been successfully translocated to Tawharanui Regional Park, which is a c. 500-ha peninsula of protected habitat that is adjacent to farmland and bush remnants.
Low (isolated)
These sites are unconnected and isolated from other suitable habitat, with the nearest suitable habitat further away than the species is prepared to travel.
Factor 2: Habitat size
Why is habitat size important?
The size of the habitat at your release site must be suitable for all stages of the life-cycle of your translocated species and sufficiently large to enable rapid population growth.
The required area will depend on the species you are translocating. For example, 200 ha of good island habitat could support 1000 North Island saddleback, whereas 1000 kōkako would require 4000 ha of good-quality habitat. By contrast, other species, such as lizards and invertebrates, can reach high abundances in much smaller sites (< 1 ha); and seabirds, which primarily forage in marine environments and only use land for nesting, can also become established in small, well-protected breeding sites.
Habitat sizes
Large
Large areas of habitat can support very big populations that have a greater probability of surviving chance events (e.g. severe weather, predator or disease irruptions) and maintaining high genetic diversity.
Medium
Medium areas of habitat can retain large populations but there may be a gradual loss of genetic diversity over time in some species.
Small
Small areas of habitat can sustain viable populations. However, these populations will be more prone to chance events (e.g. severe weather, predator or disease irruptions) and more likely to lose genetic diversity over time.
Note: There is an underlying assumption that whatever the size of the habitat at your release site, it is high quality and includes sufficient predator control [Link to the Factor 3:Minimum predator Control page].
Factor 3: Minimum predator control
Why is predator control important?
The control of predators will increase the survival of your translocated species and likely will be the critical determinant of whether your translocation succeeds or fails. Predator control can also strongly influence the growth rate of your population, affecting its long-term persistence and genetic diversity.
Some level of control of exotic mammalian predators is essential for all translocations in New Zealand. However, although some translocated species require the eradication of predators or their maintenance at zero density, others can tolerate low numbers of predators. The guild of target predators will differ between translocated species as will the control and monitoring methods required.
Levels of predator control
Predators maintained at low density
Exotic mammalian predators are usually present in low numbers at a release site, sometimes as breeding populations. These will need to be maintained at levels that are appropriate for your translocated species via ongoing control. For example, rats are often present and breeding at kōkako release sites but are reduced to very low numbers prior to the breeding season. [Link to the North Island Kokako page]
Predators eradicated or reduced to zero density
Eradication is complete removal of exotic mammalian predators. No formal definition of zero density is currently available for exotic mammalian predators. However, for management purposes, it is useful to think of zero density as a contrast between suppressed exotic predator populations, i.e. those that are always present albeit at low densities, and those that are absent, i.e. they are not detected most of the time but monitoring devices (e.g. tracking tunnels, traps, bite tags, cameras) will detect animals when incursions occur.
The ongoing challenge is in detecting incursion events, as individual invading animals often behave in a very different manner from those within a population, even at low density. Therefore, it is essential that you use extensive random stratified detection networks, both on and between control devices and lines, to detect and resolve incursions by exotic mammalian predators. For example, North Island saddlebacks have been successfully translocated to Tawharanui Regional Park despite there being several rat incursions each year as there is a rapid response to these incursions resulting in the invading rats being killed – consequently, rat breeding has only been detected once within the park since 2004. [Link to the North Island saddleback page]
How to interpret population graphs
Figure 1 provides examples of the graphs that are presented on the individual species pages.
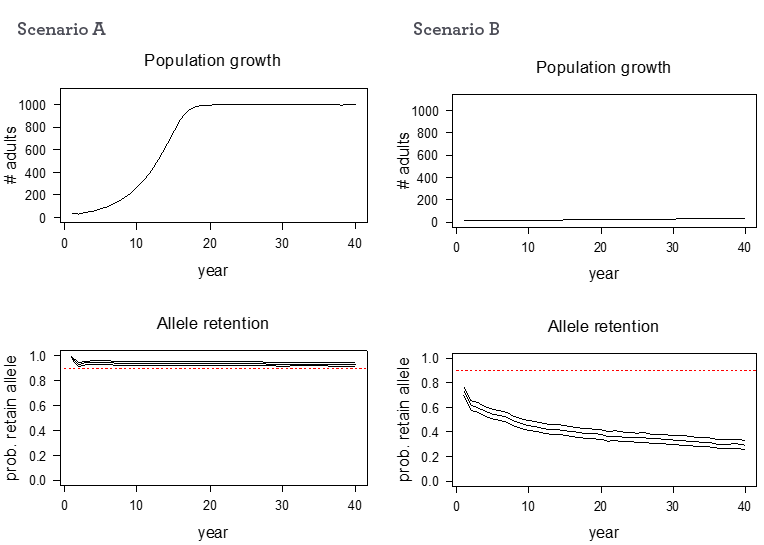
Figure 1. Examples of mohua translocation outcomes in terms of population growth (top) and allele retention (bottom). The population shown in A experienced good population growth to carrying capacity and retained high genetic diversity, whereas the population shown in B remained very small and low allele retention i.e. it lost most of its genetic diversity
Population growth graph
The top graphs in Fig. 1 show a projection of the change in total population size (y-axis) over time in years (x-axis). Population growth estimates are calculated using data from other translocations as well as expert opinion, and each of the variables can change between sites and species. Therefore, it is essential that you carry out post-release monitoring to collect accurate data for your particular site.
Allelic retention graph
The bottom graphs in Fig. 1 show a projection of the probability of retaining genetic diversity over ten generations. The ideal is to stay above the red line, i.e. retain 90% of rare alleles over ten generations. Populations with less genetic diversity may still remain viable but may be less adaptable to change over time.
More about genetic diversity
Why is genetic diversity important for your translocated population?
Genetic diversity will allow your population to adapt to changes in the environment, e.g. the emergence of a novel disease, climate change or habitat change. Although there are many viable and persistent populations with low genetic diversity, they may be less resilient to changes in their environment.
What is allele retention?
The different forms of a gene are called alleles. Therefore, allele retention can more simply be described as genetic diversity. The aim is to keep examples of all of the different genes/alleles in your population. You can measure allele retention by calculating how many rare alleles are still present after ten generations. You should aim for 90% allele retention to ensure that your population is genetically diverse and has the ability to adapt to change, making it more likely to persist in the long term. Consideration of allele retention when planning your translocation will give you the opportunity to see how you can maximise genetic diversity in the population you will establish.
Real life examples will not be as simplistic as this
To really understand what will happen at your site, you will need data from your site or a similar site. The data presented here have been collated from many sites. Therefore, although the broad generalisations will assist with your decision making, none of the results should be considered definitive.
Monitoring your own population and reporting the findings will improve the accuracy of this information and will improve the success of future translocations.